One part of the solution to the Deep State Conundrum: A State-wide Open Source Medical Data and Analytics System
As a medical professional, trust is the most important currency you have; but the Covid-19 era has forever destroyed that trust
A Statewide Open Source Medical Data and Analytics System with Independent Management
I’ll be transforming to the bill that implements Act 60/Bill S.399, called S.915, after this interruption. I will be referring to this post throughout my review of S.915.
This post provides a necessary, but probably not sufficient part of resolving the Deep State Conundrum. To this point in the discussion, I’ve primary focused on the “Constitution” argument for what the objectives of governance are. That Constitution approach by itself can not resolve the Deep State Conundrum. The world and our culture are now in a new era, kind of like going from the Triassic to the Cretaceous. The Deep State has advanced in power throughout the world that it must, in a real sense, experience an extinction event. The technology and ethics of the world has outpaced the ability of mankind to control it. In some ways, technology can and will, if not curbed, continue to facilitate the consolidation of power in the Deep State and its controlling oligarchs. We will truly “be happy and have nothing.”
However, technology can be used to restore the democratic process and restore a proper balance of power. The Deep State knows that, and they have built a legal foundation through this legislative framework to keep us serfs, us stupid masses, from ever counteracting their “solution” and turning the corner.
We must act now to write rewrite Title 44 and all that is in S.915 to free us from their plans.
These are the five objectives that need to be implemented to counter the Deep State Conundrum:
1. All regulations, executive orders, and executive branch specifications of any kind must be considered laws and, as required by the Constitution, laws can only be established by the legislature. Therefore, the legislature must have an above-boards, open to all citizens, way to review and pass all these specifications as laws. (This could be the standard bill process or a separate workflow that is more efficient.)
2. All committees must be chaired by a PMI certified project manager who may be a domain expert, but may not have monetary interest in the domain of the committee. There must be members on the committee from suppliers, stakeholders, and consumers associated with the domain. The stakeholders must include public citizens who have no relation to the suppliers and stakeholders and can not have been lobbyists from the domain, as well as legislators from the relevant legislative committees.
3. The entire public healthcare industry and government entities must be consumers of a statewide, independent of both the healthcare industry and healthcare government entities, integrated medical database system as defined in S.975 with amendments. This database system must be open source so any citizen in the world can perform analyses on it. There should be no other database the government public health entities use for analysis and reporting.
4. Conditions can not be written into Federal funding related to rewarding or punishing receiving healthcare entities for following protocols or prescribing pharmaceuticals. All healthcare facility funding from the federal government or pharmaceutical or health equipment companies to state healthcare facilities must be provided to the legislature and must be part of the legislature's public record.
5. Consumers must be protected from indemnified pharmaceutical and healthcare products.
Evaluating the priority of these five steps, I come up with this:
1. Regulations, executive orders, etc. How can these be evaluated? Sound data, uncontrolled by the Deep State.
2. Committee activities. How can they function with sound decisions? Sound data, uncontrolled by the Deep State.
3. This is the source of the sound data, uncontrolled by the deep state
4. How to evaluate the conditions written into Federal funding? Sound data, uncontrolled by the Deep State.
5. How how consumers protected from uninsured products and provided with the data necessary to make an informed decision on whether or not to take a pharmaceutical? Sound data, uncontrolled by the deep state.
The most important step we can take is a proper design of a statewide medical database and the management organization that will control it. All other medical freedoms and, it’s not too strong a position to take, all other medical freedoms depend on writing into law a particular database design and management organization.
They WILL be building a statewide database. It is specified in Bill S.915. There is no “IF” about it. We can not let them design it or control it.
I have already provided enough information to create a bill that meets all the requirements of this database. I created a substack article back in November, 2023 with the complete strategy for funding, planning, and developing the database. and planning process.
A South Carolina State-wide Database (RHIO + HIT) for near-real time analytics of anonymized health data from around the state
The following is the outline for a bill that will be filed for the Spring 2024 session of the South Carolina legislature. It should have a high priority due to its high return on investment and low risk assuming rapid passage of the bill. The Problem
https://aletheiatheyounger.substack.com/p/a-south-carolina-state-wide-database
I wrote another post that provided a draft bill to implement the concepts in the previous article.
A bill filed at the state level to create a health information database and integrated analytics toolkit
I’ve written about this in detail before:
https://aletheiatheyounger.substack.com/p/a-bill-filed-at-the-state-level-to
I have a number of new followers, so I am repeating that bill below, inline this time, rather than as a pdf, which is included in the above post.
Before I present it, I would like to emphasize a key dependency of this approach. If it is to be successful, a very large percentage of the citizens in the state must have their medical information in the database. In South Carolina, as far as I know, all the major hospitals, which serve almost all citizens, use EPIC as their EMR. As a first and very significant step, the patient records database can be built from exports from the EPIC databases. EPIC provides tools that implement aggregating medical records from multiple EPIC databases. EPIC also provides a suite of analytical tools for that database. This means that the vast majority of records can be brought “on-line” to quality production level in a relatively short time, certainly in less time than it will take to create the organization, the SCHIO, that will manage it. In the articles above I do mention that I have the primary members of the team “assembled,” all renowned experts in their discipline and who meet the standards of the bill. Still, even after the 2024 elections, the SC General Assembly will be controlled by the Uniparty, so it will be an uphill battle, but a battle we must win. If the plans of the manufacturers to ramp up mRNA “vaccines” is anywhere near true, the citizens of South Carolina and the world are going to see a sustained effort exercising the psyOps of the Covid-19 era to get the “vaccines” in the bodies of the world’s citizens. Though a fraction of the population has “caught on” to the psyOps tricks, the majority is still in the fear and follow stage. This database will be an essential barrier to protect us from mandates and them from the destructive components of the “vaccines.”
Ultimately, to include all medical records without compromising privacy, I think this solution should require every citizen to have a healthcare professional associated with the medical records. This role is call the Primary Care Physician (PCP) in most insurance plans and in the health industry. But that role could be anyone that can sign and enter records into an EMR.
Also, ultimately, the EMRs used in some medical clinics, and even in other health venues, such as physical therapy, will need to be linked to the statewide database. Several companies provide production tools to map medical databases of one EMR to another EMR. The state will need to fund creating those maps, or purchase existing maps.
The following bill draft is the one most important action the state can take to regain trust when it is involved in medical affairs. Your comments on improving this will be greatly appreciated.
Summary
To amend the South Carolina code of laws by enacting the “South Carolina Health Information Analytics Act” by adding chapter 140 to title 44 so as to create and SUPPORT AN OPERATIONAL permanent advisory organization, “the South Carolina Health Information Organization (SCHIO) , AND a health information database with a robust toolkit, “the South Carolina Medical Information and Analytics System,” with supporting constraints usage protocols, and dependencies
Title: South Carolina Health Information Analytics Act
Section 44-140-10. Principles
(A) Independence. Ensure that potential biases and potential conflicts of interest are minimized, balanced, or otherwise managed in the design and implementation of all processes, practices, and policies related to the Act.
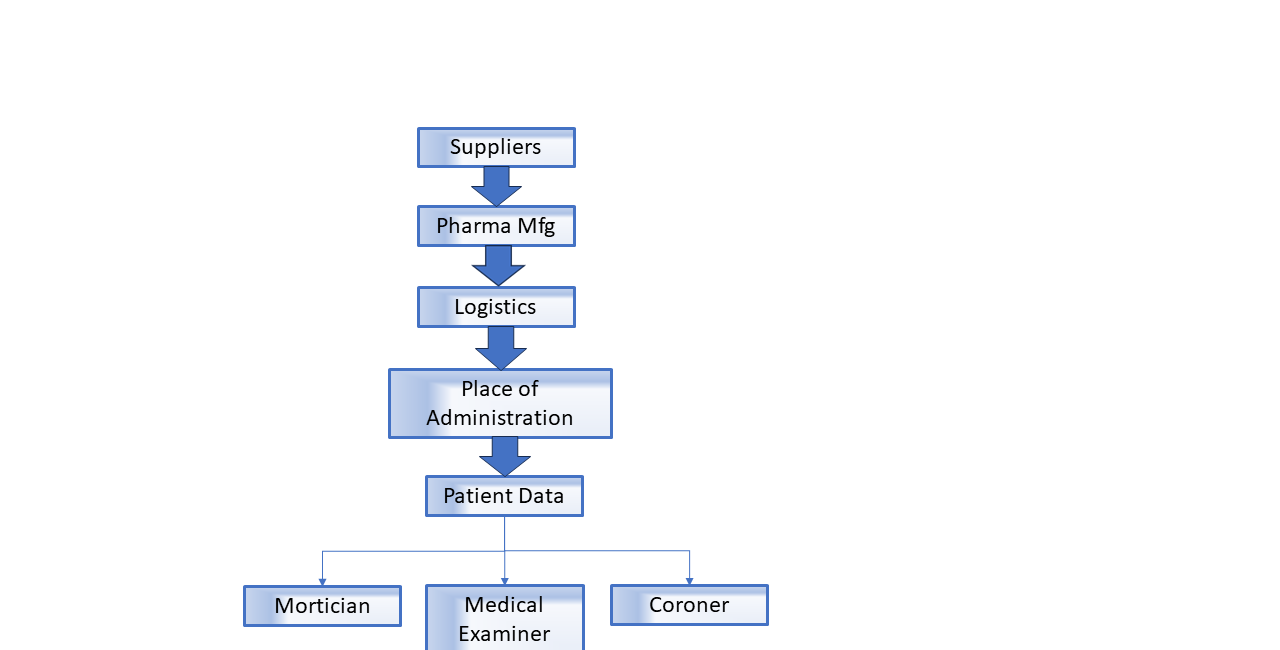
(B) Integration. In order to establish the cause of symptoms and adverse effects, the Covid-19 era has demonstrated that healthcare data must include (1) the analysis of the pharmaceutical product for purity and functionality (2) the evaluation of the quality of the manufacturing process (3) full access to a patient’s health history, including all pharmaceuticals given to the patient, along with the dates, lot information, and place of administration, (4) mortician, medical examiner, and autopsy results for designated infectious diseases
(C) Functionality. A full suite of analytic tools, from those commonly used to experimental, must be integrated into the system.
(D) Transparency. All processes, practices, and policies related to the healthcare data are developed in the spirit of openness, shall be clearly articulated, and shall be available to interested persons or entitie; any deviations from them must be documented and justified.
(E) Fairness. All processes, practices, and policies related to the databases are designed and implemented in a fair manner.
(F) Protection of confidentiality. The design and implementation of the the healthcare database shall protect the confidentiality of individually identifiable information while enabling traceback to patients when adverse effects are detected that should be addressed by other patients that have the same medical situation
(G) Timeliness. New snapshots of data should be available at least on a weekly basis in order to capture early signals of adverse effects as well as new disease outbreaks.
Section 44-140‑20: Objectives
For the purposes of this chapter, the objectives are:
Re-establish integrity and trust in public health in South Carolina.
Remove the dependency on the federal government and transparently provide data and analysis support for pharmaceutical effectiveness and adverse effects in support of public health in South Carolina.
Establish a executive body and a healthcare database of integrity that will be free of influence from any government agency or any healthcare organization.
Establish a culture in the medical community that questions whether health problems are associated with pharmaceuticals as either unintended consequences or adverse effects which threatens public health. Provide funding to study and identify these threats.
Have access to reliable and timely health data to protect the citizens from Constitutional overreach by the federal government and manipulation, incompetence, and poor policy recommendations regarding health and education by the executive branch of state government. Additional state initiatives are necessary to ensure that citizens of all ages can critically assess information in general, but especially information related to their own and their family’s health.
To protect SC citizens from Corporate and Institutional overreach, manipulation, incompetence, and poor policy recommendations regarding health.
To protect the informed consent ethic, and basic human rights
Section 44-140-30: Definitions
For the purposes of the chapter:
“SCHIO” means a governance agency called the South Carolina Health Information Organization. It’s overall characteristics will be that of a RHIO: “A health information organization that brings together health scientists, database analysts, program managers, manufacturing experts, to establish a healthcare database within a defined geographic area and governs health information aggregation and analytics tool integration in a secure environment for that database, all for the purpose of improving health and care in that region.”
“HIT” means Health Information Technology. See https://www.hhs.gov/hipaa/for-professionals/special-topics/health-information-technology/index.html
“SCHIAS” means South Carolina Health Information and Analysis System.
“Designated pharmaceutical” is a pharmaceutical that has been designated by the SCHIO as requiring analysis, evaluation, tracking, or other actions to understand and assure pharmaceutical or protocol quality, effectiveness, or adverse effects with respect to a particular infectious disease or human organ malady.
“Stakeholder” in this endeavor is the South Carolina citizen; it is not any healthcare organization or government entity, with the exception that the medical committees of the South Carolina Medical committees are the representatives of the stakeholder.
“Program Manager” is a role in the SCHIO and is a professional, PMI certified project manager. The individual in this role is not required to have experience with projects in the healthcare domain, but is required to have experience with complex projects where the individual human participants and organizations have little project management maturity as defined by the PMI.
“Program Dependencies” are entities that this Act depends on for successful implementation of this Act. These include, but aren’t limited to hospital systems that have implemented EPIC for patient records; mortuaries, County Coroner offices, and pharmaceutical manufacturers and their suppliers, and locations that sell or administer target pharmaceuticals.
Section 44-140-30: South Carolina Health Information Organization
The South Carolina Health Information Organization (SCHIO) shall be established to create and manage a health information technology (HIT) database and infrastructure and all policies related to its lifecycle, standards, use, and contents
SCHIO shall report directly to the Secretary of Health and Policy (44-12-20, Bill 915)
SCHIO membership shall
A. Not include anyone who has or has had a business financial relationship with a pharmaceutical company.
B. Not include anyone who has a current relationship with a healthcare organization
C. Include one member from the House 3M, Medical Affairs subcommittee
D. Include one member from the Senate Medical Affairs committee
E. Include a PMI certified Program Manager that does not have and has not had a relationship with the South Carolina Department of Administration or other state organization
F. Include a Senior Biologics Director from the University of South Carolina who’s demonstrated skill has been the analysis and characterization of pharmaceuticals in general and the n1-ΨxRNA/LPN pharmaceuticals in particular.
G. Include a Senior Manufacturing Quality Director whose professional experience includes auditing pharmaceutical manufacturing facilities, including suppliers and logistics, for Good Manufacturing Practice.
H. Include a Senior Scientific Director with expertise in biostatistics and operations research, whose skill is recognized world-wide, and who has not been an advocate of the failed Covid-19 management strategies of the world, federal, or state healthcare organizations
I. Include a Senior Technology Director from Clemson who is a member of the Electrical and Computer Engineering Department with skills in informatics, cyber systems, security, and artificial intelligence in healthcare.
J. Include two recognized analysts who have demonstrated skill in analyzing Covid-19 data for adverse effects and communicating those analyses using web dashboards.
K. Include a Senior Pathologist with experience in infectious disease pathology
L. Include a representative from the hospital systems of in the state
M. Include a representative of the County Coroners of the state
N. Include a representative of the embalmers of the state
O. Include a representative of the medical examiners of the state
P. Include a public communications specialist without ties to the pharmaceutical or healthcare industries or the SC government
Q. Include a representative from the SC Public Health Department who has not directed any of the DHEC response during the Covid-19 era
R. Include a staff representative of the Governor’s office who has not been involved in the governor’s office response during the Covid-19 era
Each member shall be nominated and appointed by consensus of the House and Senate Medical Committees for a period of three years, with an opportunity for one renewal.
The SCHIO shall have public meetings each quarter to report on progress and analytical results
The SCHIO shall establish priorities for all data extraction protocols based on the historical health record and future infectious and/or pharmaceutical development predictions
The SCHIO shall establish priorities for design and development
The SCHIO will receive funding through the annual budget process and will provide Financial Management for itself, with oversight by the Office of the Secretary of Health and Policy)
Section 44-140-50: The South Carolina Health Information and Analysis System
A Health Information Database (HIT), called the South Carolina Health Information and Analysis System (SCHIAS), will be designed, established, and maintained according to the guidance of the SCHIO.
The database will be an integration of the 5 databases described in Sections 44-140-60, 44-140-70, 44-140-80, 44-140-90, and 44-140-100.
The analysis system will provide access to the integrated database and be comprised of analytic tools commonly used for healthcare and manufacturing data analysis as well as various prototype and experimental tools.
All Program Dependencies must supply data requested by the SCHIO.
The database will be maintained by a central information technology organization associated with Clemson University that has expertise in complex, cyber-secure systems.
The system will add data on a weekly basis from the various databases described in 44-140-40-B. Extreme security will be employed to assure the data can not be corrupted, destroyed or manipulated during transfer.
The system will archive data after three years and the archive will retain data for a period designated by the SCHIO.
The system will be open access after registration by any user. Qualifications for use will be determined by the SCHIO, but access should not be restricted to healthcare organizations and government agencies; individuals with relevant interest must be allowed complete access.
Section 44-140-60: Manufacturing Lot Quality and Logistics History Database
This database contains the quality and logistics history data for every lot of a designated pharmaceutical administered to or used by South Carolinians.
These data must be supplied by any pharmaceutical manufacturer distributing a designated pharmaceutical in South Carolina or by the governmental organization responsible for logistics.
These data must be supplied to the administrator of this database by any pharmaceutical manufacturer of a designated pharmaceutical if it is administered to a patient while out-of-state.
Section 44-140-70: Manufacturing Vial Quality Sampling and Control Database
The Manufacturing Vial Quality Sampling data consists of the results of quality sampling of vials or other packaging implementation by SCHIO designated quality assurance organizations
Sampling can be at various locations in the logistics process of the designated pharmaceutical, as determined by the SCHIO, but must include sampling vials at the point of administration to a patient.
The sampling procedure must implement a quality sampling process that dynamically adjusts the sampling quantity of product at a location and at other locations, depending on the results.
Sampling protocols must include designated pharmaceutical product “halt distribution” and “recall” protocols, the details of which are defined by the SCHIO.
Sampling and quality services should be contractor selected by the SCHIO.
Section 44-140-80: Manufacturing Good Quality Management Database
The data in this database consists of the results of good quality management audit reports of pharmaceutical manufacturers as designated by the SCHIO.
The format, audit protocols, and details of the data required are as designated by the SCHIO
The audit shall be performed by the SCHIO or by a selected contractor.
Section 44-140-90: Patient EMR Database
These data consist of ICD codes and patient notes, and time designations as defined by the SCHIO for a designated pharmaceutical.
Data shall include all information on the administration of the designated pharmaceutical to the patient.
Data should include all informed consent forms along with the information provided for consent at the time the patient signed the form. If the data are not available in the EPIC database, then the SCHIO shall establish a process for obtaining said data and entering it into this database.
The patient is anonymized but can be back-traced to the attending physician related to the patient note associated with an examination.
The data shall be provided by every healthcare facility in the state operating an EMR. (The state shall provide funding to maintain a translation to the standard format required by the SCHIAS.)
Section 44-140-100: Post Mortem Database
The data in this database come from the following sources, each location of which shall provide the data to the database administrator in a cyber-secure manner for any patient who died after administration of designated pharmaceuticals or protocols and had ICD code sequences and/or combinations or death certificate entries designated by SCHIO
Embalmer data: procedure and data defined by the SCHIO for the designated pharmaceutical or protocol
Medical examiner data: procedure and data defined by the SCHIO for the designated pharmaceutical or protocol
Coroner data: procedure and data defined by the SCHIO for the designated pharmaceutical or protocol
Section 44-14—110: VA Databases
Veterans’ Administration Medical Databases shall be integrated directly or indirectly into the SCHIAS
This ends the language of the proposal. There are various assumptions, issues, and dependencies that should be discussed and resolved:
The necessary “traceback mechanism” that allows the public health department, a healthcare professional, or registered analyst to determine the patient belonging to a data record is that a healthcare provider must be registered with a medical record. This means every patient encounter must be associated with a healthcare provider. This means that every healthcare provider must provide a mechanism where the provider transfers ownership of all medical records under that provider’s responsibility to another provider when that provider is no longer providing healthcare in South Carolina. This is a dependency that must be agreed to by the healthcare community.